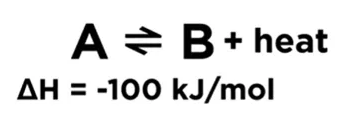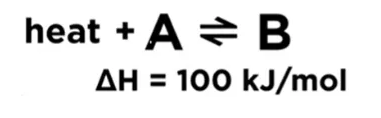A-level化学-化学平衡-精讲!
2020-02-22
今天小学诚请到了我们化学组组长,来给同学们讲讲什么是A-level化学的化学平衡,一起来学习吧~
什么是化学平衡?
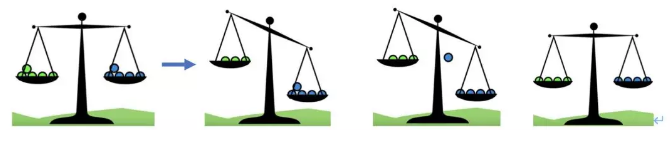
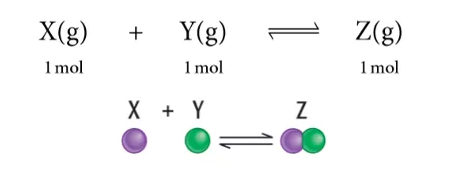
化学平衡是指在宏观条件一定的可逆反应中,化学反应正逆反应速率相等,反应物和生成物各组分为常量浓度的状态。我们也称其为动态平衡 (dynamic equilibrium), dynamic 在这里是指forward和backward的reaction会同时发生。根据Le Chatelier’s Principal可以用来看一些external conditions对equilibrium的影响,主要包括Temperture, Concentration, and Pressure.
Le Chatelier’s principle states that if an external condition is changed the equilibrium will shift to oppose the change (and try to reverse it).
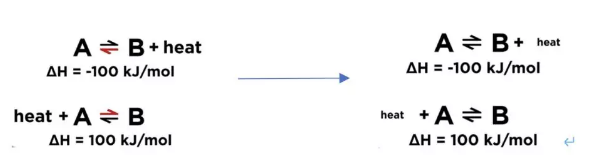
If increasing the concentration on one side, the position of equilibrium will shift to the opposite side to decrease the formation of molecules of that side and balance out the extra concentration. If decreasing the concentration on one side, the position of equilibrium will shift to this side and increase the formation of molecules of this side to compensate the concentration lost. 首先我们需要figure out 这个反应是exothermic 还是endothermic reaction. 1) Exothermic reaction; releasing energy in terms of heat. 2) Endothermic reaction; absorbing energy in terms of heat. If increasing the temperature, which means more heat. The position of equilibrium will shift to the other side and use up some excess heat. 如果我们decrease temperature 的话,同样equilibrium会转移到heat 减少的那一边,去保存更多的能量 According to the Le Chatelier’s Principal, if the pressure of system has been increased, the position of equilibrium shifts in the direction of fewer gas molecules to minimize this increase. 更多的Z 分子生成了,减少了X和Y分子的形成,所以分子总数会减少,从而系统的压力便会减少。反之亦然,外界条件使pressure 减少,那么equilibrium shift to the side with more molecules. 从而去增加pressure回到reaction 之前的状态。 怎么样,同学们看懂了吗?如果还有疑问,试听课走起!