今天我们和学诚国际教育丁老师一起来看看A-level化学关于卤代烃类物质性质的问题。
一、物质性质
1. CH3Cl, CH3Br, C2H5Cl 在常温常压下是气体. 其他的卤代烃多为液体, 且物质的质量越大, 其沸点越高. are all gases at room temperature and pressure. The other haloalkanes are liquids with boiling points related to molar mass.
2. 卤代烃不溶于水. They are all immiscible with water.
3. 卤代烃类物质通式为CnH2n+1X (where X = halogen atom)
4. 由于卤素较强的电负性, 因此卤代烃多为极性分子. Haloalkanes are polar due to the inductive effect of the halogen atom. Since the halogens are more electronegative than carbon, they have a greater share of the electrons in the C-X bond.
示意图如下:
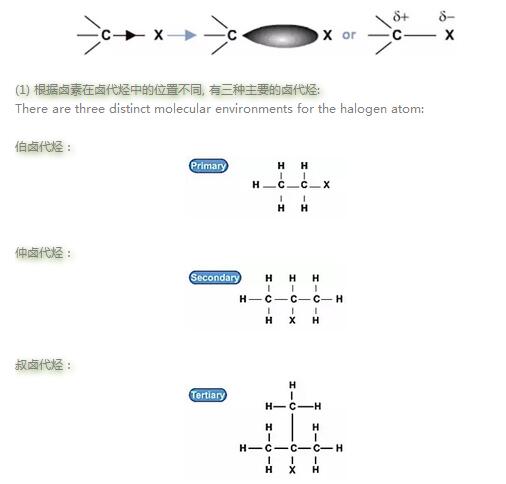
极性趋势: 叔位的卤代烃>仲卤代烃>伯位的卤代烃
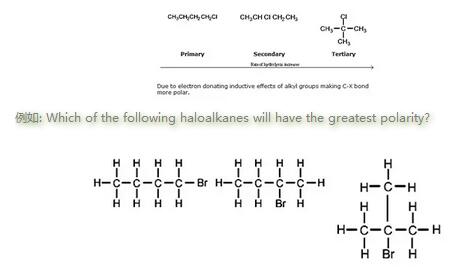
The third example (tertiary halogenoalkane) has the greatest polarity. This is because the positive carbon ion (carbocation) is stabilised by the inductive effect of the three other bound carbons.
机理分析: 卤素的电负性偏大, 因此卤代烃的比同根的烃类物质更活泼. The polarity of the C-X bond results in haloalkanes being much more reactive than their parent alkanes. Therefore they are of greater importance industrially.
二、化学性质
1. C-X 键上可发生的反应Types of reaction occurring at C-X bond:
a. Nucleophilic substitution reactions亲核取代
b. Elimination reactions消去反应
(1) Nucleophilic substitution
卤素的电负性比碳元素大,吸引电子能力更强, 导致碳元素偏正电. 因此,碳原子会被亲核试剂攻击, 使得反应发生. The inductive effect of the halogen atom results in a positive charge on the carbon atom to which it is attached. Hence, this carbon atom is readily attacked by nucleophiles.
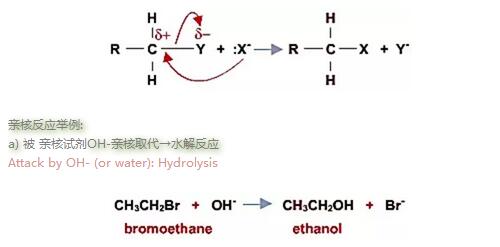
亲核反应举例:
a) 被 亲核试剂OH-亲核取代→水解反应
Attack by OH- (or water): Hydrolysis
卤代烃在碱性溶液中易于发生水解反应, 纯水中反应非常缓慢. 在使用氢氧化钠/氢氧化钾水溶液回流条件下, 速度变慢,但是产率更高. The haloalkanes are attacked only slowly by water. The rate is much faster, but a poor yield is obtained if the haloalkane is refluxed with aqueous sodium or potassium hydroxide.

b) 被亲核试剂氰基攻击:Formation of nitriles: attack by CN-
使用氰化钾的乙醇溶液回流,可产生If a haloalkanes is refluxed with an alcoholic solution of KCN, an acid nitrile is formed: R-C=N
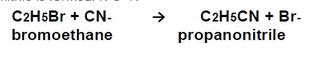
c) 被亲核试剂氨攻击,产物为胺类物质. Attack by ammonia NH3: Products are amines.
使用加热条件下的氨乙醇溶液回流, 会产生胺和铵盐混合物. If a haloalkane is heated with an alcoholic solution of ammonia in a sealed tube, a mixture of products is formed. The mixture consists of amines and amine salts.
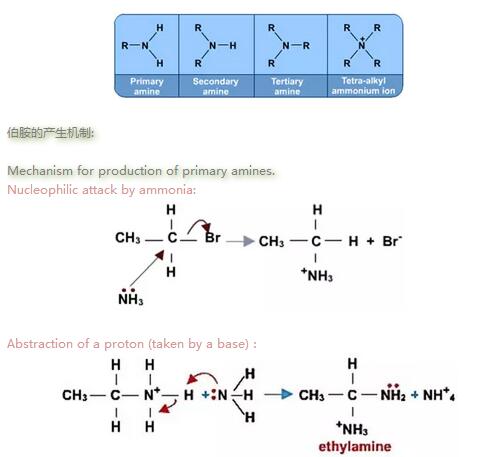
Nucleophilic attack by ammonia:
Abstraction of a proton (taken by a base):
(2)消去反应Elimination reactions
碱性条件下回流可产生醇类物质. When haloalkanes are heated with aqueous solution of potassium or sodium hydroxide, the major product is the alcohol, produced by nucleophilic displacement of the halogen by OH-.
加热条件下, 使用浓缩的氢氧化钠醇溶液回流, 可在 C-X 键的位置发生消去反应, 脱去 HX 小分子, 产生 C=C 双键. If the reaction conditions are changed so that the haloalkane is heated with concentrated alcoholic potassium hydroxide, the major product is an alkene due to the elimination of hydrogen halide.
学诚国际教育针对学生的不同年级和不同基础,提供了最丰富的选择;班课和一对一混合学习,科学合理,符合中国学生学习习惯。

